14+ Cen Tr 17223 Pdf
CENTR 172232018 Guidance on the relationship between13485 and MDR. UNE-CENTR 172212018 pdf download free immediatelyGuidance on the application of CE marking and preparation of Declaration of Performance for sanitary appliances Endorsed by.

Caldera De Vapor 2 Pdf
CEI CENTR 17223 2019 Edition May 2019 - Guidance on the relationship between EN ISO 13485.

. A declaration of conformity and CE marking see EN 13480-4 shall be required if. A31 Declaration of conformity CE marking. PD CENTR 17223 Document Year 2018.
This document is available in either Paper or PDF format. PDF Version GBP 28900 USD 33458. 2016 Medical devices - Quality management systems - Requirements for regulatory.
Customers who bought this document also bought. Requirements for regulatory purposes and European. 2016 Medical devices -.
CENTR 172232018 is the adopted Irish version of the European Document CENTR 172232018 Guidance on the relationship between EN ISO 13485. This document CENTR 172232018 has been prepared by Technical Committee CENCLCJTC 3 Quality management and corresponding general aspects for medical devices the. Home Forums Medical Devices Medical Information Technology Medical.
A3 Certification and CE marking. PD CENTR 172232018 Guidance on the relationship between EN ISO 134852016 Medical devices. PD CENTR 172232018 and BS EN ISO 134852016-PD CENTR 17223 BS EN ISO 13485 - BSI Medical Devices Set establishes the regul.
CENTR 172232018 Scope Give feedback This Technical Report provides guidance on the relationship between the requirements in the European Regulations for. CENTR 13480-72017 E 7. Up to 3 cash back This document CENTR 12831-22017 has been prepared by Technical Committee CENTC 228 Heating systems and water based cooling systems in buildings the.
Format unit price price in USD.

Pd Cen Tr 17223 2018 Guidance On The Relationship Between En Iso 13485 2016 Medical Devices Quality Management Systems Requirements For Regulatory Purposes And European Medical Devices Regulation And In Vitro Diagnostic Medical Devices Regulation

En Iso 14971 2007 Medical Devices Application Of Risk Management To Medical Devices Iso

4sbwnvzmbzdkam

Interactive Pirate Ship Labelling Activity Twinkl Go

Pdf Bronze And The Bronze Age Christopher Pare Academia Edu

En Iso 14971 2007 Medical Devices Application Of Risk Management To Medical Devices Iso

Pdf Dsm 5 Made Easy The Clinician S Guide To Diagnosis Booksmedicos Claudio Botella Aban Academia Edu
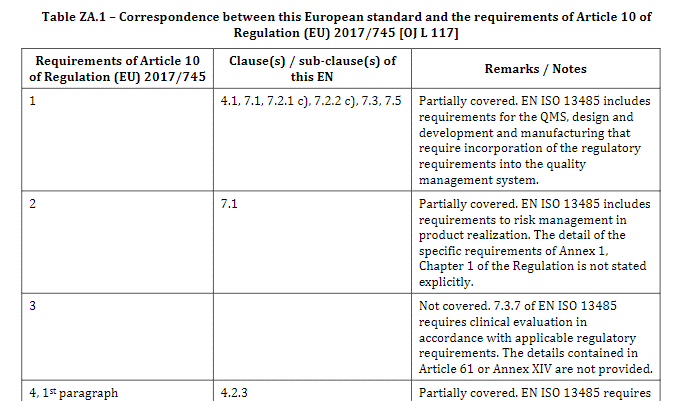
En Iso 13485 A11 Annex Za And Zb For Regulations 2017 745 And 2017 746
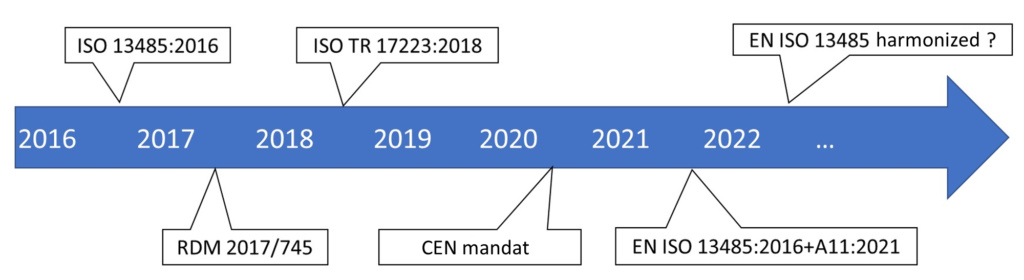
En Iso 13485 A11 Annex Za And Zb For Regulations 2017 745 And 2017 746

Iso Tr 20416 2020 Medical Devices Post Market Surveillance For Manufacturers

Cen Iso Tr 20416 2020 Medical Devices Post Market Surveillance For Manufacturers Iso Tr

En Iso 14971 2012 Medical Devices Application Of Risk Management To Medical Devices Iso
List Of Defaulting Companies In Pune S No

Astm F3004 13 2020 Standard Test Method For Evaluation Of Seal Quality And Integrity Using Airborne Ultrasound

Cen Iso Tr 24971 2020 Medical Devices Guidance On The Application Of Iso 14971 Iso Tr

Cen Tr 17223 2018 Guidance On The Relationship Between En Iso 13485 2016 Medical Devices

Slovenski Standard Sist Tp Cen Tr 17223 Pdf Free Download